My research on pore-scale relative permeability uses 3D timeseries x-ray microtomography combined with in situ flow to look into the evolving fluid distributions during multiphase immiscible fluid coinjection.
Here we investigate two fundamental questions:
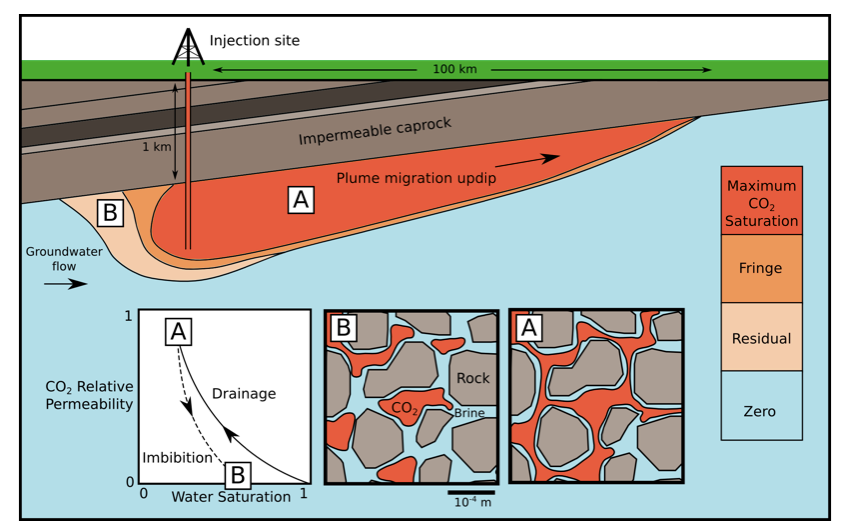
The conceptual model of relative permeability when carbon dioxide is injected into a deep reservior. Figure credit: Dr Catriona A. Reynolds
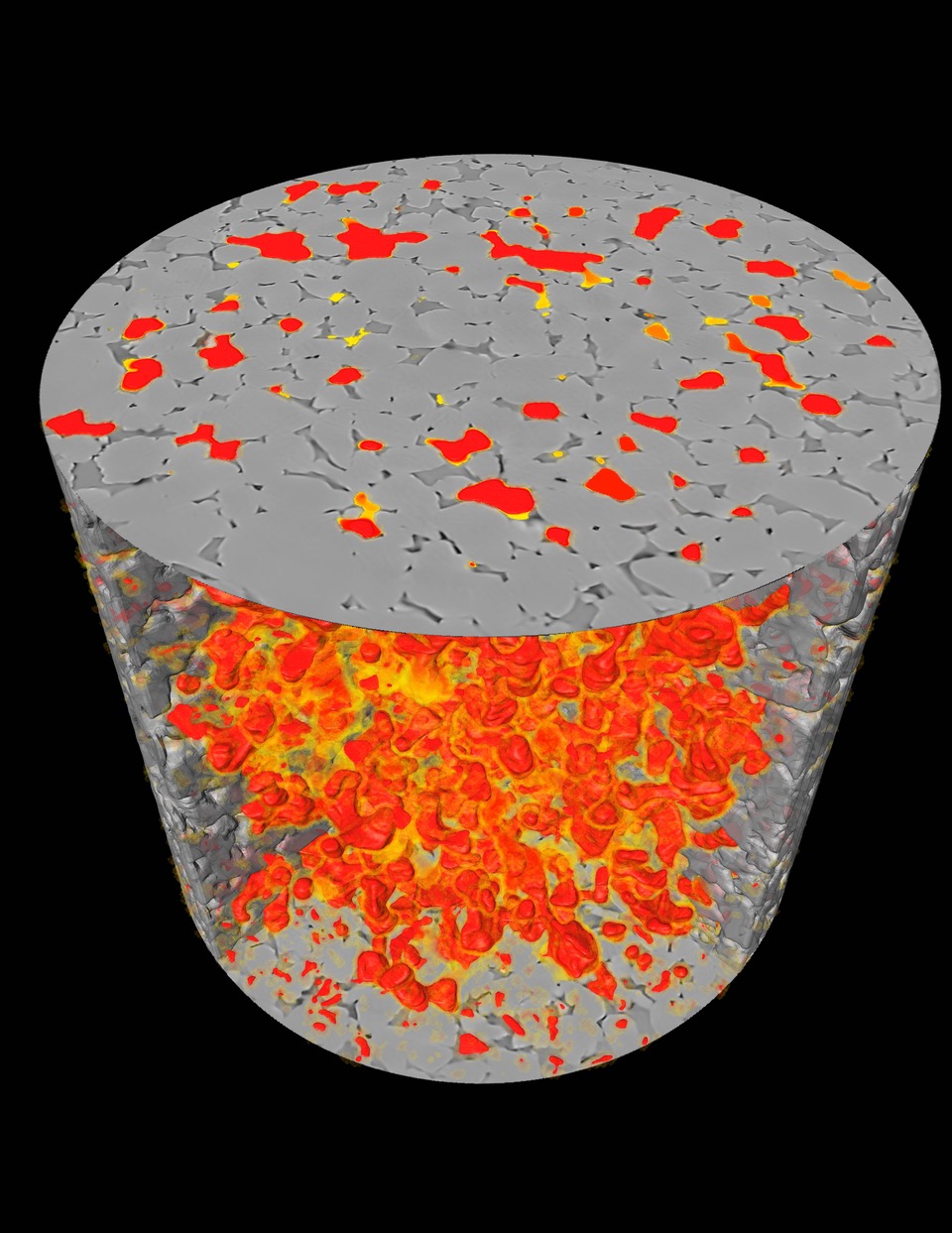
3D rendering of 4D non-weeting phase connectivity during steady state flow.
Authors: C.A. Reynolds, H.P. Menke, M.G. Andrew, M.J. Blunt, S.C. Krevor
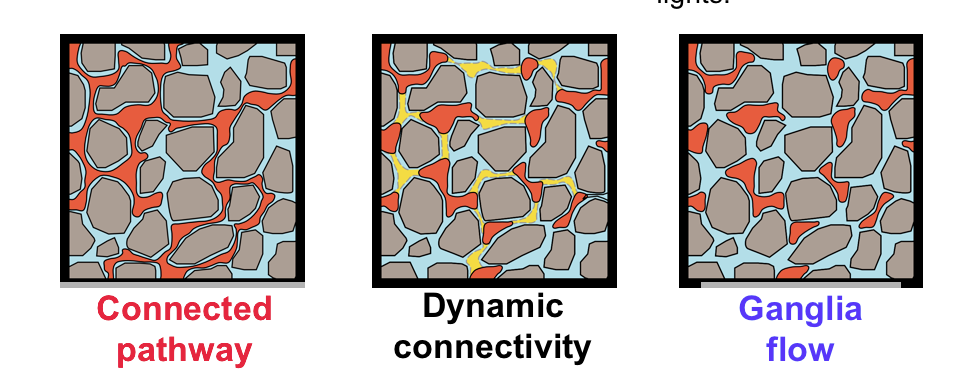
Abstract: The current conceptual picture of steady-state multiphase Darcy flow in porous media is that the fluid phases organize into separate flow pathways with stable interfaces. Here we demonstrate a previously unobserved type of steady-state flow behavior, which we term "dynamic connectivity," using fast pore-scale X-ray imaging. We image the flow of N2 and brine through a permeable sandstone at subsurface reservoir conditions, and low capillary numbers, and at constant fluid saturation. At any instant, the network of pores filled with the nonwetting phase is not necessarily connected. Flow occurs along pathways that periodically reconnect, like cars controlled by traffic lights. This behavior is consistent with an energy balance, where some of the energy of the injected fluids is sporadically converted to create new interfaces.
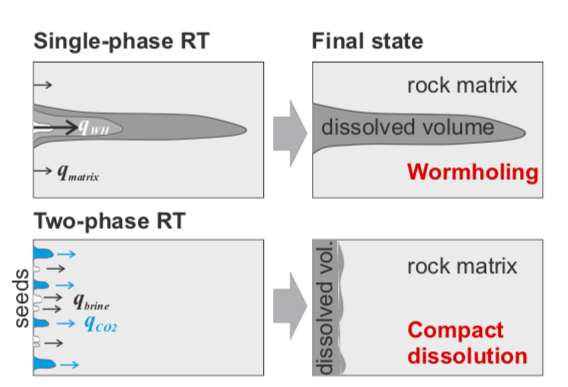
Model of dissolution regimes. Upper images: wormholing regime in single phase flow. Lower row: coinjection of CO2 and brine. Non-wetting CO2 occupies the low-pC WH seeds of the dissolution structure and suppresses further growth, leading to compact dissolution. Figure Credit: Ott & Oedei 2015 Geophyiscal Research Letters.
So how does the presence of this additional gas phase in the center of the pore-space affect the flow of the acidic brine? How does the reaction rate and pattern change? Preliminary results from Ott and Oedie GRL 2015 (left) on core plugs using low resolution medical CT indicate a dramatic change in dissolution regime.
The aim of this project is to investigate combined reaction and steady-state flow at the pore scale to understand the controlling mechanism behind these regime changes.